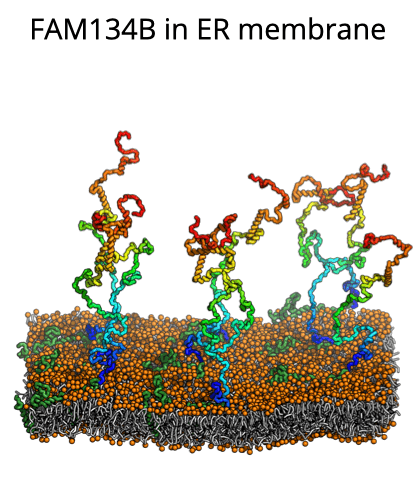
Until now, the involvement of intrinsic disorder in membrane shaping processes has not been fully understood. A multidisciplinary group around IBC2 Team Leader Ram Bhaskara now reports in PNAS detailed insights into how intrinsically disordered regions (IDRs) in proteins help remodel cellular structures, particularly within the membrane proteins of the endoplasmic reticulum (ER). Focusing on the ER-phagy receptor FAM134B, they explored how membrane-anchored disordered protein regions interact with membranes. Through advanced computer modeling and molecular dynamics (MD) simulations, the team found that – depending on context – these highly flexible regions exhibit different behaviors: Driven by their conformational entropy alone, they can sense and induce membrane curvature, thereby aiding in local remodeling. However, when combined with membrane-shaping elements like the Reticulon homology domain (RHD), they amplify large-scale remodeling processes by active scaffolding. This "Janus-like" behavior is sequence-encoded and shared among other proteins involved in ER-phagy. It allows IDRs to boost protein clustering and accelerate the reshaping of the ER, providing a fresh perspective on their role in regulation of membrane dynamics and shaping of cellular organelles during autophagy. The in silico predictions based on this model is validated by newly developed functional assays. The study is the result of a collaboration between the Bhaskara team and the groups of Ivan Đikić at IBC2 and Gerhard Hummer at Max Planck Institute of Biophysics.
Link to publication: https://www.pnas.org/doi/10.1073/pnas.2408071121